Articles
Approaching green ammonia as a zero-carbon fertilizer, fuel and energy storer
Special Focus: Advances in H2 technology
S. SAKTHIVEL, Tata Consulting Engineers Ltd., Mumbai, India
In addition to its established role in the fertilizer industry, ammonia has many energy applications: it shows potential as a zero-carbon fuel, a low-carbon energy storage medium and a carrier for H2. Despite the promise it holds for a low-carbon future, however, ammonia is primarily produced via integration of steam methane reforming or coal gasification/fuel oil oxidation (to produce H2) with the Haber-Bosch process. This process releases more carbon dioxide (CO2) into the atmosphere than any other chemical synthesis process: it emits 1.6 metric t–1.9 metric t of CO2 per metric t of NH3 produced, accounting for ~1.3% of global CO2 emissions each year. Decarbonization of this process is highly desirable—to meet the Paris Agreement’s 1.5°C target, global emissions must fall 7.6% every year for the next decade. Energy producers are therefore aligning to develop and deploy carbon-free processes and/or products which lead to decarbonization in the near future. Decarbonization options mainly revolve around increasing energy efficiency, moving industries towards the use of clean energy and reducing demand for CO2 emitters. The last of these three tactics does not apply here, as demand for ammonia is not falling but rising; adoption of green ammonia production processes, however, could help achieve lower emissions and lead to decarbonization of the fertilizer industry.
AMMONIA: APPLICATIONS AND PRODUCTION
Ammonia is extensively used for the following: fertilizer production, air-conditioning and refrigeration of large building units, explosives manufacturing, textiles, the pharmaceutical industry and as an absorption agent in acid gas removal. Further emerging uses for ammonia include: as an energy source in power generation, whether by direct combustion in gas turbines or as cracked ammonia in alkaline and proton exchange membrane (PEM) fuel cells; as fuel for use in direct combustion engines, solid oxide fuel cells or PEM fuel cells; and as a medium for heat transfer.
Like H2 and methanol, ammonia is considered a chemical energy storage substance. It can be used to store large quantities of energy over long time periods, and to facilitate distribution of that energy. Ammonia is liquefied by cooling to –33°C at atmospheric pressure or by increasing pressure to ~10 bar at room temperature. Liquid ammonia possesses a high H2 gravimetric density of 17.8% by weight, along with a volumetric H2 density of ~108 kg/m3 and an energy density of 13.77 MJ/L at 20°C and 8.6 bar. H2’s volumetric energy density is 0.01 MJ/L at ambient temperature and pressure, 0.2 MJ/L at 17 bar, 1.5 MJ/L at 350 bar, and 9.98 MJ/L at –253°C. Liquid ammonia has a 38% higher energy rate than liquid H2. It is also comparatively more stable and convenient to transport.
Ammonia, including liquid ammonia, has a well-established and high-capacity infrastructure for production and distribution, including pipelines, tank trucks, bunker shipping, and more. It also has well-defined regulations and a good safety history spanning more than a century. From a safety perspective, ammonia is far less flammable in air than natural gas, methanol, H2 or gasoline vapors; while it is considerably more toxic than the aforementioned substances, safety protocols and best practices regarding its handling are already well established in the relevant industries.
Globally, ammonia is predominantly produced at commercial scale via the Haber-Bosch process, which produces ammonia from atmospheric nitrogen (N2) and H2 using a metal catalyst at high temperature and pressure. This H2 is extracted from fossil fuels through steam reforming of natural gas, coal gasification and partial oxidation of heavy oil. Around 65% of global ammonia production, including much of what is manufactured by fertilizer companies in India, uses H2 from natural gas; 97% of ammonia production in China uses H2 from coal gasification.
The Haber-Bosch process consumes 8 MWh of energy per metric t of ammonia produced, with the natural gas reforming process for H2 production (where applicable) accounting for 75% of the total energy demand. The remaining 25% is consumed during ammonia synthesis, gas compression and ammonia separation. The H2 generation process also accounts for 90% of CO2 emissions involved in the Haber-Bosch process. The only pathway to achieve deep decarbonization is the use of H2 sourced from renewable electrical sources, otherwise known as green H2.
An overview of the ammonia color code.
Although ammonia is a colorless gas at room temperature, as with H2 it is common to hear it referred to by color. As illustrated by FIG. 1 and expounded upon below, these colors correspond to different methods of producing ammonia.
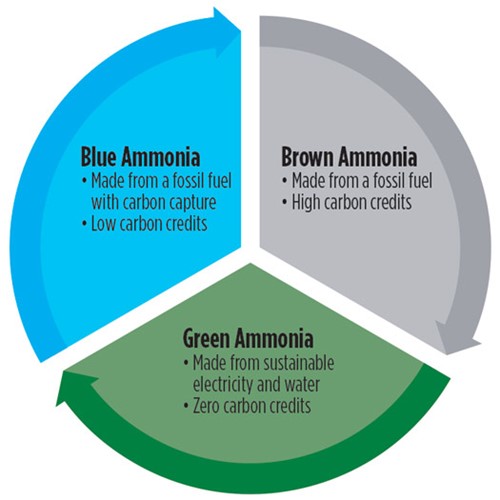
FIG. 1. Color-coded types of ammonia.
Brown ammonia is produced via the Haber-Bosch process from gray or brown H2, both of which use fossil fuels as feedstock. Gray H2 is extracted from natural gas by means of steam reforming, while brown H2 is created via coal gasification. Both colors of H2 emit CO2 during production and release it into the atmosphere, as does the Haber-Bosch process itself. Brown ammonia accounts for most of the ammonia produced since the Haber-Bosch process was developed.
Blue ammonia is produced through the same methods as brown ammonia, but carbon capture and storage is integrated with the traditional process to avoid CO2 escaping to the atmosphere.
Green ammonia is produced using renewable, CO2-free energy sources, and correspondingly without the use of hydrocarbons. The H2 it uses as its prime raw material is produced via water electrolysis using 100% sustainable electricity sources. Green ammonia production also requires air separation units (ASUs) to produce N2. FIG. 2 depicts the production and applications of green ammonia.
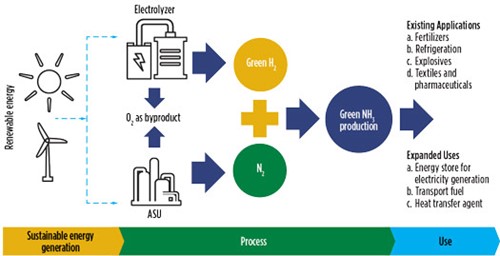
FIG. 2. Green ammonia production routes and their applications.
Green ammonia in depth.
A standard green ammonia production unit comprises three modules:
- H2 generation and supply unit: This generates H2 via water electrolysis, and is suitable for the storage and handling of H2 and its co-product of O2, which is released into the atmosphere
- N2 generation and supply unit: This generates N2 via an ASU and is suitable for the storage and handling of N2
- Ammonia production and storage: This synthesizes ammonia, as well as handling its storage.
A typical large-scale green ammonia production plant consumes 10 MWh–14 MWh of energy per metric t of green ammonia produced. While this represents greater proportional energy consumption than seen in brown H2, the cost is offset by the environmental benefits (i.e., lower emissions). Despite evidence of growing interesta, further research and development are still required to lower the cost and improve the efficiency of green H2-related technologies.
Challenges and mitigation.
The production, storage, distribution and transportation of green H2 all represent key challenges to green ammonia production. The transportation of low-density fuel such as H2 is very difficult and may require new or modified infrastructure developments, thus adding additional costs to H2 production. Transportation problems also include delivery costs, H2 purity and minimizing H2 leakage.
To mitigate these challenges, de-centralized H2 production could be introduced into the fertilizer, methanol, iron and refining industries, all of which require large quantities of H2. These industries would install electrolyzers within their boundary limits that utilize electricity generated by solar and/or wind plants via a power grid. This renewable electricity is transportable through existing power transmission networks and would then be used to produce H2 onsite, doing away with the need to transport it. H2 storage would remain necessary, however, as ammonia production requires round-the-clock availability of H2. Therefore, an electrolyzer capable of producing more H2 than needed should be installed, and the excess H2 temporarily stored onsite for use when renewable electricity is not available.
Takeaway.
Ammonia production is presently a large contributor to global CO2 emissions due to its dependence on fossil fuels. The major energy requirements, along with most of the CO2 emissions, occur due to the H2 production process upon which ammonia synthesis relies. Green ammonia is therefore only feasible if H2 production is made fully dependent on renewable energy sources. With that condition met, ammonia is a carbon-free alternative to hydrocarbons as an agent for carrying and storing energy. Existing technologies, infrastructure and policies make ammonia convenient and safe to transport, and technologies for green ammonia production are undergoing rapid development due to increased research into energy efficiency. Areas of further research in the H2 supply chain include cost reduction and better performance for electrolyzers, H2 storage and H2 transportation infrastructure.H2T
NOTES
a Examples of green ammonia projects currently under construction include a 4-GW green ammonia plant in Saudi Arabia by NEOM, Air Products and ACWA Power; the H2U Eyre Peninsula Gateway Hydrogen Project in Australia; an engineering and procurement contract signed by CF Industries Holdings, Inc. with thyssenkrupp for the Company’s green ammonia project in its Donaldson, Louisiana (U.S.) plant; and Yara International ASA’s plan to build a renewable H2 plant in Norway.
S. SAKTHIVEL is a Senior Technologist at the Technology Group of Tata Consulting Engineers Ltd. in Mumbai, India. His primary focus is on green chemicals, green fuels, the energy transition and decarbonization, with the responsibility for evaluating emerging technologies and commercialization. He has experience in process engineering; technology analysis, screening and selection; techno-economic analysis; pilot setups; process hazard analysis; basic, applied and market research; and powder science and technology. He has published several papers in national and peer-reviewed international journals.

