Articles
Clean H2 and e-fuels: Important pillars of the energy transition
Special Focus: Advances in H2 technology
S. DIEZINGER, Siemens Energy, Nuremberg, Germany
Global energy consumption is projected to rise nearly 50% above its current level over the next three decades.1 Meeting this demand while mitigating the effects of climate change will require a diverse range of energy sources that includes both hydrocarbons—particularly natural gas—and renewables. Coexistence, not competition, will be vital in driving a successful energy transition while ensuring a reliable energy supply.
H2 will play an important role. According to the International Renewable Energy Agency (IRENA), H2 and its derivatives could represent as much as 12% of final energy consumption by 2050.2 Blue H2 will account for much of the near-term growth in H2 production capacity; despite this, achieving net zero CO2 emissions by 2050 will require industry and governments to develop innovative approaches to zero-emissions green H2, as well.
Green H2 has become increasingly competitive with its gray and blue counterparts in recent years, a development primarily attributable to a continuing decline in the cost of renewable energy generation. As several real-world projects have now shown, e-fuels (synthetic fuels made via renewable energy) derived from green H2 can be produced at prices comparable to, or even lower than, those of biofuels given the right combination of partners, technologies and climatic conditions. The economics of producing these derivatives have therefore become more attractive, unlocking opportunities for sector coupling and decarbonization.
The case for e-fuels. In addition to a carbon footprint 90% lower than that of fossil fuels, e-fuels have the potential to outperform biofuels in both CO2 avoidance and production costs. Finally, e-fuels have advantages when it comes to land usage and water consumption.
As FIG. 1 shows, several decarbonization use cases exist for e-fuels produced from green H2. In the mobility sector, for example, e-methanol can be blended with either conventional fuels or biofuels to reduce the mixture’s overall carbon intensity, thus reducing emissions in automotive, marine and aviation applications. Fully sustainable e-gasoline can then be produced using methanol-to-gasoline synthesis, as can other products such as olefins, formic acid, formaldehyde and a number of widely used fuel additives. Additionally, well-established processes exist for producing e-ammonia by combining green H2 with nitrogen (N2) obtained via air separation. End uses for ammonia include feedstock for fertilizer (e.g., urea and ammonia phosphates) and as a synthetic fuel for the shipping industry; it is also an excellent H2 carrier over long distances using existing infrastructure, possessing a density of 177 kg H2 per metric t of ammonia.
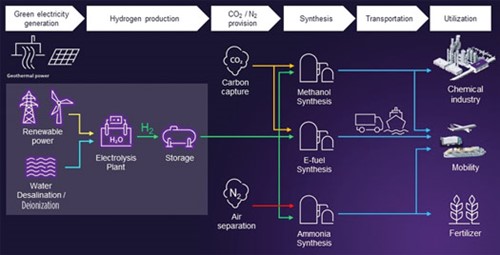
FIG. 1. Green H2: From production to end-use applications.
DEVELOPING THE WORLD’S FIRST INTEGRATED E-FUEL PLANT
Several green H2 plants are under development across the globe. One of these, the Haru Oni project, is sited in Chile—the country’s low electricity prices and favorable climatic conditions make it ideal for green H2 production. This project will use low-cost, green wind power generated in Chile’s Magallanes province to produce CO2-neutral fuel and will be the world’s first integrated, large-scale commercial plant to produce methanol-based e-gasoline.
The project’s pilot plant is expected to produce approximately 130,000 l (liters) of e-fuel in 2022. Two subsequent expansions are planned: production capacity should reach 55 MMlpy by 2024 and 550 MMlpy by 2026.
Project technologies.
At the heart of the facility will be a proton exchange membrane (PEM) electrolyzer. The PEM is permeable to protons but not to gases. Once the electrolyzer uses electricity generated by the plant’s wind turbines to break water (H2O) into O2 and H2, the PEM will prevent the two product gases from mixing. The splitting of water molecules will occur via electrodes connected to the voltage source’s positive and negative poles and sited on the front and back of the membrane. The electrolyzer will not require preheating before being switched on or off, making it well suited for the load profiles of renewable power sources such as wind and solar, which are volatile by nature.
Downstream of the electrolyzer, the green H2 will be combined with CO2 filtered from the air to produce synthetic methanol. This, in turn, will be converted into e-gasoline, providing a decarbonization solution for mobility applications.
Project economics.
While levelized cost of energy (LCoE) has a greater impact than any other variable on the economics of green H2 and e-fuel production, the capacity factor (full-load hours) of the electrolyzer used takes a strong second place—it defines the capital efficiency of the electrolysis and synthesis plant. With a favorable LCoE of $20/MWh and 6,000 full-load hr availability for some locations, green H2 can already compete with gray and blue H2 produced from steam-methane reforming or autothermal reforming of natural gas (see FIG. 2). Since the costs for green H2 also affects the production costs for derivatives in regions such as Chile, the prices for e-fuels are lower than for other environmentally sustainable fuels used in the mobility sector, including ethanol.
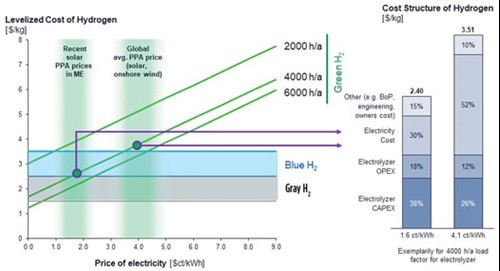
For carbon-based e-fuels such as e-methanol, the cost of CO2 also impacts project economics. Currently, the most efficient and cost-effective method of carbon capture utilizes industrial sites with high-CO2 concentration flue gases; this is particularly the case when oxy fuels are combusted. As was previously mentioned, the Haru Oni project will utilize direct air capture (DAC)—it will capture CO2 from the atmosphere. The particular challenge of DAC lies in the high energy consumption required to capture CO2 given its low concentration in the atmosphere (around 440 ppm). Industrial-scale business cases for e-fuel production are only viable when both electricity and CO2 costs are favorable.
As illustrated in FIG. 3, with a favorable LCoE and cost for CO2 supply, e-methanol and e-ammonia can be produced at almost the same cost as their gray counterparts. In the case of e-methanol, production costs are within the range of biofuel.
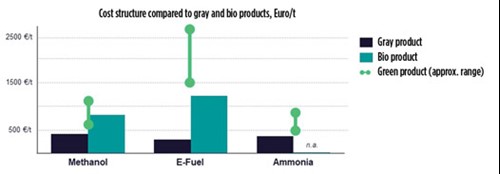
FIG. 3. Given favorable conditions, e-methanol and e-ammonia can compete with gray and bio counterparts.
Takeaway.
The current major obstacle to the accelerated build-out of green H2 production is the availability of clean electricity. In Europe alone, it is estimated that 1,100 GW–1,300 GW of dedicated renewable generating capacity and up to 550 GW of electrolyzer capacity will be needed to fulfill the green H2 demand forecasted by 2050.3 Current trends are heartening. Despite the pandemic, the world managed to add a record 260 GW of renewable energy capacity in 2020, more than four times the additional capacity from other sources. By 2030, according to IRENA, global renewable energy capacity could reach 10,770 GW, nearly quadruple the current capacity.
H2 is positioned to become an essential component of a decarbonized world. While approximately 95% of H2 produced today is gray, this is changing as blue and green production capacity rises. There is still a long way to go, but the question is no longer if energy transition can be accomplished but rather how quickly.H2T
NOTES
aAssuming 50-MW electrolyzer, WACC 8.9%, CAPEX electrolyzer 640 $/kW, electrolyzer efficiency 75%, 20-yr lifetime, OPEX 5% of CAPEX (including exchange of electrolyzer module).
LITERATURE CITED
1 U.S. Energy Information Administration (EIA), International Energy Outlook 2019, September 2019, online: https://www.eia.gov/outlooks/ieo/pdf/ieo2019.pdf
2 International Renewable Energy Agency (IRENA), World Energy Transitions Outlook: 1.5(degree)C Pathway, June 2021, online: file:///C:/Users/Lee.Nichols/Downloads/IRENA_World_Energy_Transitions_Outlook_2021.pdf
3 Goldman Sachs, Equity Research. “Green hydrogen: the next transformational driver of the utilities industry,” September 2020, online: https://www.goldmansachs.com/insights/pages/gs-research/green-hydrogen/report.pdf
 STEFAN DIEZINGER is the Vice President of Sustainable Energy Systems at Siemens Energy. In his current capacity, he works with companies to assess, develop and implement decarbonization strategies. Prior to the spinoff of Siemens Energy, Dr. Diezinger held several executive sales positions with Siemens AG, including Vice President of Sales: Industrial Business. He has also worked in project management and engineering capacities. Dr. Diezinger earned a Bch degree in business administration from the University of California at Berkley, an MS degree in process engineering and a Ph.D. in mechanical engineering, both from FAU Erlangen-Nürnberg.
STEFAN DIEZINGER is the Vice President of Sustainable Energy Systems at Siemens Energy. In his current capacity, he works with companies to assess, develop and implement decarbonization strategies. Prior to the spinoff of Siemens Energy, Dr. Diezinger held several executive sales positions with Siemens AG, including Vice President of Sales: Industrial Business. He has also worked in project management and engineering capacities. Dr. Diezinger earned a Bch degree in business administration from the University of California at Berkley, an MS degree in process engineering and a Ph.D. in mechanical engineering, both from FAU Erlangen-Nürnberg.

