News
H2 fuel risk assessment and differing views of ignitability
M. MOOSEMILLER, Baker Engineering and Risk Consultants Inc., Chicago, Illinois and J. K. THOMAS, Baker Engineering and Risk Consultants Inc., San Antonio, Texas
Hydrogen is being contemplated or implemented as an alternative fuel in several parts of the world. Creating a large-scale infrastructure for H2 fuels requires an objective assessment of the associated risks. Such an assessment requires consideration of the probability of ignition of potential releases in a variety of storage, loading and fueling situations.
In researching a book1 on estimating ignition probabilities for releases of flammables into the atmosphere, industry subject matter experts (SMEs) were solicited for their experience-based opinions on a range of hypothetical release/ignition situations. Reasonable agreement was achieved among the SMEs for most fuels. However, in the case of H2 releases, there was a large divergence in opinion ranging from near-zero expectation of ignition to 100% ignition probability for the same event. A corresponding divergence in ignition probability models was noted by Jallais.2
This broad span in H2 ignition probability opinions suggested that either the SMEs were not as expert as was believed (although they were), or that there were underlying factors that led to such varying experiences among the SMEs. In fact, the literature published within the past 15 yr suggests that physical phenomena may be involved in H2 releases that might reasonably result in these differing experiences.
This article is intended to provide an unbiased literature review on the subject, as well as suggest how this literature and ongoing H2 field testing might provide consistent and more accurate expectations for ignition probabilities that can be used with confidence to evaluate H2 fuel risks.
H2 ignition properties and factors. Mechanisms proposed for igniting H2 include those shown in FIG. 1. However, even this list may not be exhaustive, as indicated by Gummer and Hawksworth’s work through 2007,3 which indicated that the proposed mechanisms do not explain some reported H2 ignitions (or non-ignitions). In addition, these mechanisms are not universally agreed upon, or in some cases may not be significant enough to cause ignition in H2 fuel applications.
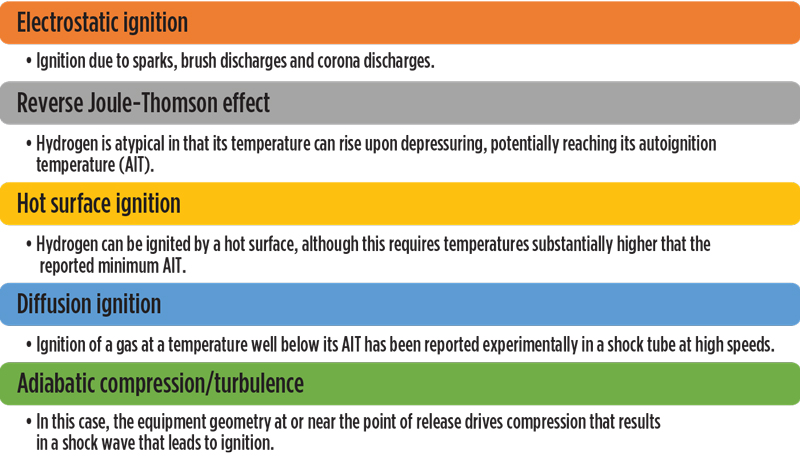
Fig. 1. Hydrogen ignition mechanisms.
As noted earlier, SMEs had widely different views of H2 ignitability based on H2 release events experienced during their careers. As an example, the survey participants were asked to provide their estimates for the probability of ignition for an H2 leak under the following conditions:
- Temperature = 100°F (38°C)
- Pressure = 100psig(7 bar)
- Hole size = 1-in. (25-mm) diameter
- Release duration = 100 sec
- Weather = Typical daytime conditions
- Release = Standard Class I Division 2 process area.
The respondents were asked to estimate the likelihood of immediate ignition of H2 (at or near the source) and delayed ignition (ignition after a cloud had developed). Their responses are provided in TABLE 1.
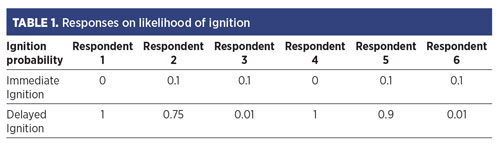
Considering that the responses for the ignition probabilities of most hydrocarbon fuel release scenarios were in much better agreement, it appears that the SMEs were biased due to their prior experiences with H2, which appear quite diverse. Clearly, there must be some variable(s) that were not accounted for in the release conditions provided to the survey participants, which the SMEs implicitly accounted for or assumed.
Anecdotal record of hydrogen ignition events. Gummer and Hawksworth3 noted that “Studies undertaken many years ago on H2 vents […] showed that ignition was rare during fine weather, but was more frequent during thunderstorms, sleet, falling snow, and on cold frosty nights.” This suggests that for some events, weather conditions may be a factor.
In addition, the following specific passages from Gummer and Hawksworth3 suggest that a static discharge mechanism may be involved in some ignitions:
The 1922 incident investigation
After several spontaneous ignitions of hydrogen at 2.1 MPa being discharged to atmosphere had been reported, work was undertaken to determine the cause. Various experiments were undertaken on discharging hydrogen to atmosphere, but no ignitions occurred despite discharging through many different types of nozzle made from differing materials. However, cylinders had been noted for having quantities of iron oxide (rust) in them […] Therefore, mixtures of hydrogen and oxygen were stored at an initial pressure of 1.1 MPa at various temperatures in the presence of iron oxide to determine whether the oxide catalysed the reaction [...] at temperatures above ambient, the pressure slowly fell, indicating that the oxidation reaction was occurring. The times were about 24 hours at 100°C, nine hours at 200°C, and one hour at 380°C. There was no explosion at any time.
Subsequent experiments on discharging hydrogen into an open funnel fitted with a long pipe showed no ignitions except when the funnel was obstructed by an iron cap […] when the hydrogen leaked out of a flange—the corona discharge was visible, which increased when the pipe was tapped to stir up dust. An ignition followed after the tapping. Further work showed that when sharpened copper wires were used to promote corona discharges, ignition occurred when the point was bent away from the gas direction, whereas no ignition occurred when the wire was pointing in the direction of flow. Consequently, it is apparent that a corona discharge was likely to have been the source of ignition in this case.3
The 1926 and 1930 incidents and experiments
The second explosion occurred when the isolation valve between a pressurised pipeline and a chromium plated vessel was opened to depressurise the line from about 4.9 MPa. The explosion occurred immediately […] It was noticed that there was ample evidence of fine dust, presumably metal oxide, being present in the pipe-work during the examination after the explosion. This led Fenning and Cotton to surmise that the explosion had been initiated by an electrostatic discharge, presumed to have been generated by the fine dust being blown along the pipe by the high-velocity hydrogen. However, despite many attempts, no ignition was achieved in their experiments.3
Incidents reported by Bond
Bond reports two incidents, sourced from a private conversation, where hydrogen ignited. In the first incident, hydrogen at a pressure of 11.1 MPa was leaking from a gasket between two flanges. The hydrogen had not ignited at the time when the fitters arrived to tighten the bolts. It was reported that on the second strike of the hammer wrench that was being used to tighten the bolts, there was an ignition. It is not apparent whether the ignition source was an impact spark from a hammer wrench being used to tighten the bolts on the joint, or attributed to the mechanism of a diffusion ignition. The second incident refers to a cylinder of hydrogen being connected to a piece of laboratory apparatus. The laboratory technician cracked the valve open to clear any dirt out of the connection, and when he did so, the escaping gas ignited immediately. Bond attributes this ignition to the phenomenon of diffusion ignition.3
Jackass Flats Incident, 1964
This incident […] involved the deliberate release of a large quantity of hydrogen to determine the sound pressure levels. The hydrogen was released from storage at an initial pressure of 23.6 MPa and an initial rate of 54.4 kg s-1, for a period of 10 seconds […] In the run where the gas was not deliberately ignited, after 10 seconds, the 150 mm diameter valve was being closed, and three seconds after starting to close the valve, ignition occurred.3
Gummer and Hawksworth3 summarized the work through 2007 by saying that the proposed mechanisms do not account for all the reported ignitions (or non-ignitions) of H2; however, particulates and/or static appear to be a factor in several of the reported cases. They cite specific incidents in which ignitions occurred in an obstructed discharge but not in unobstructed discharges. This is similar to what was observed by Dryer et al.4 and Hooker et al.,5 and was attributed to the relative presence or absence of the formation of turbulence near the point of release and may be symptomatic of the adiabatic compression mechanism.
Factory Mutual6 analyzed reported H2 fire and explosion events from 1961–1977 for the U.S. government. TABLE 2 shows the findings.
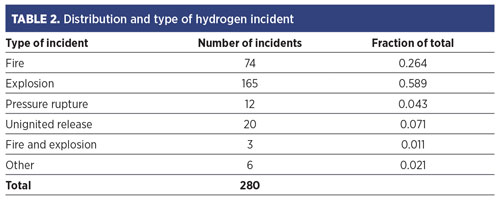
The extent of any data bias is unknown. One might expect that unignited events are underreported relative to fire events, and perhaps fire events are underreported compared to explosions. The recorded ignition sources are identified in FIG. 2. The “unknown” category is large. This could simply be indicative of an incomplete reporting or lack of knowledge by the reporter; however, it could also reflect the presence of unfamiliar ignition mechanisms.
While most of this article describes releases of gaseous H2, there is one record of tests with liquid H2 by Witcofski.7 The focus of Witcofski was on dispersions, not ignitions; so ignition was not desired, and one can assume that no obvious ignition sources were allowed in the area. The author notes that no ignitions occurred during the tests of seven liquid H2 releases, each of 5.7-m3 volume.7
Thomas et al.8 provided a review of unconfined H2 vapor cloud explosions (VCEs) that included incidents beyond those discussed here. More recent incidents included those at the Silver Eagle refinery and Muskingum River power plant. H2 VCEs reported following the publication of Thomas et al.8 include incidents at an H2 fuel station in Norway (2019) and at the OneH2 facility in North Carolina (2020).
Experimental record of H2 ignitions. Recent papers and presentations by the UK Health and Safety Executive or affiliated agencies9 have explored possible H2 ignition mechanisms experimentally and through literature review. In some cases, the conclusions are not clear. Hooker et al.5 evaluated ignitions in a cavity downstream of a bursting rupture disk; their data is summarized in FIG. 3. There is no obvious trend in this data, suggesting that factors other than source pressure are involved.
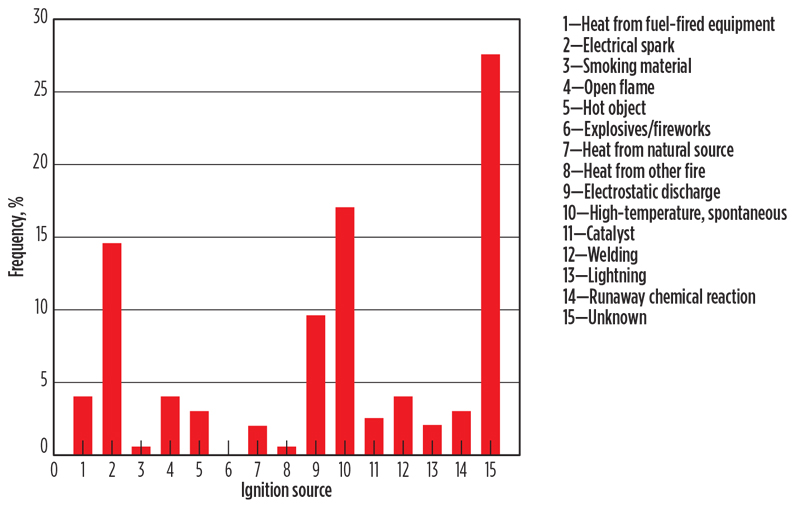
Fig. 2. Hydrogen incident statistics, 1961–1977, distribution by ignition source.
Swain et al.10 experimentally investigated the ignition of H2 releases. The results from this study suggested that the effects of increased pressure on increasing ignition probability (as relates to static discharge potential or increased overall flowrate) may not be as great as one might expect:
- Releases at Mach 0.1 velocity ignited farther away from the source than releases of the same nominal flowrate (using a smaller-diameter orifice) at Mach 0.2
- The concentration of H2at the farther distance where ignition took place was approximately 7 vol% at Mach 0.1, but 10 vol% at Mach 0.2.
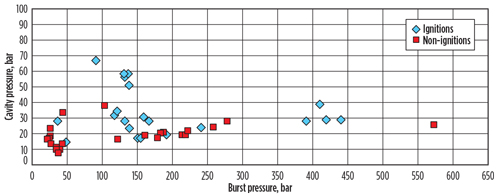
Fig. 3. Plot of cavity pressure against burst pressure for cases of ignition and non-ignition.
Since it is unlikely that there will be ‘strong’ ignition sources, such as a fired heater in an operating plant at the distances used in the study (approximately 5 ft), this may suggest that the dominant mode of H2 jet ignitions near the source may be static. It may also indicate that the probability of delayed ignition (and not just the probability of explosion) may be related to the degree of congestion/confinement in the surrounding plant.
Dryer et al.4 performed a series of experiments with H2 in which the presence of obstructions or confinement at the point of release was found to influence the odds of ignition. The ignition phenomenon was observed only for release pressures greater than 200 psig. The requisite confinement was effective down to a discharge piping length of approximately 1.5 in., below which ignition did not occur. Ignition also disappeared at lengths greater than approximately 40 in., which was attributed to combustion heat removal by the piping.
Also of interest were releases into open atmospheres that did not produce ignition at release pressures as high as 800 psig. However, the implications for release of high-pressure streams with low ignition temperatures are significant—that ignition may occur in releases taking place in confined spaces (e.g., relief device discharge piping or perhaps flange leaks), but not into open spaces (e.g., a pinhole leak in a process vessel).
Grune/Kuznetsov et al.11 provided experimental evidence supporting a shock wave ignition mechanism, using a rupture disk release apparatus. Their test data is shown in FIG. 4, and photographs from their test are provided in FIG. 5.
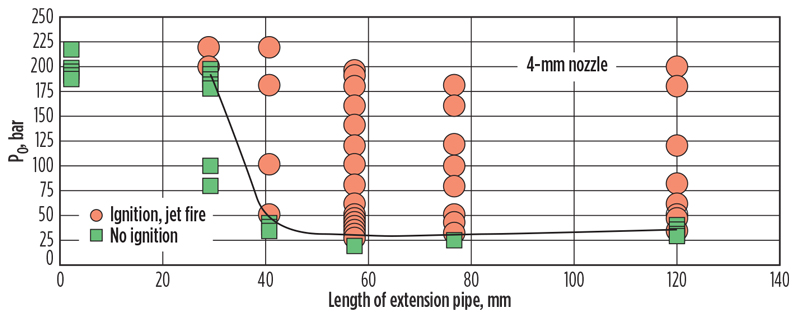
Fig. 4. Critical conditions for spontaneous ignitions inside the extension tube downstream of the rupture disc.
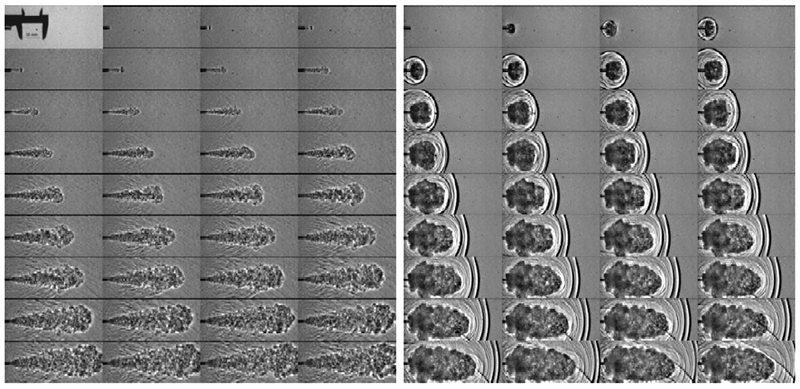
Fig. 5. Development of the H2 jet on the nozzle exit (4-mm nozzle, 200-bar initial pressure) in experiments with (right) and without (left) rupture disc in the flow path from the leak valve to the nozzle exit.
Golub et al.12 performed similar experiments accompanied by numerical simulations. Some of their results are summarized in FIG. 6. The X-axis “L” value in FIG. 6 is the length of the low-pressure chamber in their apparatus. The primary conclusions from their work were that:
- The possible reason for self-ignition is heating by the primary shock wave
- Self-ignition occurs if the source H2pressure is on the order of 150 bar–400 bar, the temperature of the H2and the air is 300K or more and the hole diameter is greater than 3 mm.
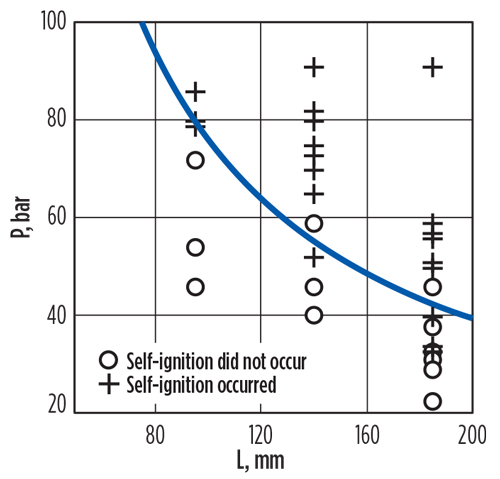
Fig. 6. Experimental results showing dependence of minimal reservoir pressure on the external tube length when self-ignition occurs.
Ongoing experimental efforts. Baker Engineering and Risk Consultants Inc. (BakerRisk) is actively addressing the unique safety challenges of H2 and the rapid growth of H2 infrastructure. As part of that support, BakerRisk is performing a series of internal testing programs focused on H2 dispersion and ignition probability. The primary goal of these tests, which are being conducted at one of BakerRisk’s test facilities, is to study the ignition probability of H2 under a range of typical operational conditions and release sizes. A secondary goal is to compare test cases of gaseous H2 releases to dispersion models to validate or improve those models, as necessary.
This series of H2 dispersion and ignition tests will be run using the BakerRisk cylinder skid dispersion rig (FIG. 7), which allows the team to disperse a variety of gases without significant modifications to existing and validated rigs. The cylinder skid dispersion rig was built with 316 stainless steel to allow for compatibility with a diverse range of materials and can withstand supply pressures up to 3,000 psig. Testing is being performed under a variety of release conditions, pressures and flowrates.

Fig. 7. The cylinder skid dispersion rig at the test facility, prior to commissioning.
Why does it matter to hydrogen infrastructure? Significant debate has emerged over the past several years about the potential magnitude of H2 release events with regard to VCEs. Testing and numerical simulations performed by BakerRisk and others have demonstrated that VCEs due to H2 releases can be more severe than with most other flammable materials (e.g., methane, propane, etc.8). Specifically, H2 is a high-reactivity fuel that is more likely to undergo a deflagration-to-detonation transition (DDT). Testing by BakerRisk in both unconfined and confined test rigs has shown that DDTs can occur with lean (i.e., non-optimal) H2-air mixtures.13,14 Numerical evaluations have been performed to examine the potential for DDTs in ambient vaporizers.15 The potential for explosions due to H2 jet releases has also been considered.16,17
BakerRisk has also undertaken separate investigations of H2 applications as a fuel source and of H2 ignition probabilities.18,19 These studies indicate that the hazards/risks of H2 usage may have been misunderstood until recent years, but that our understanding is rapidly developing.
Takeaway. Investigations over the past several years have begun to provide clarity on previous misconceptions about H2 ignition events. While a wide variety of opinions exists on H2 ignition probabilities, there is evidence supporting why these differing opinions might be occurring.20
Experiments have shown that high-pressure releases are subject to shock-induced self-ignition and that ignition under less severe release conditions may occur in the presence of particulates, such as pipe scale, or if there is an irregularity in the release path that creates a shock wave. Ongoing efforts will provide greater clarity to these issues, which is necessary for objective evaluations of the hazards and risks associated with moving to widespread usage of H2 as an alternative fuel source.
LITERATURE CITED
- CCPS, “Guidelines for determining the probability of ignition of a released flammable mass,” Center for Chemical Process Safety, American Institute of Chemical Engineers, New York, New York, 2013.
- Jallais, S., “Hydrogen ignition probabilities,” Air Liquide internal presentation, August 2010.
- Gummer, J. and S. Hawksworth, “Spontaneous ignition of hydrogen,” HSE Books, Research Report RR615, 2008.
- Dryer, F., M. Chaos, Z. Zhao, J. Stein, J. Alpert and C. Homer, “Spontaneous ignition of pressurized releases of hydrogen and natural gas into air,” Combust. Sci. and Tech., Vol. 179, 2007.
- Hooker, P., M. Royle, D. Willoughby and J. Udensi, “Self-ignition of hydrogen by various mechanisms,” Hazards XXII, Symposium Series No. 156, 2011.
- Zalosh, R., T. Short, P. Marlin and D. Coughlin, “Comparative analysis of hydrogen fire and explosion incidents, Progress Report No. 3,” for Division of Operational and Environmental Safety, U.S. Department of Energy, Contract No. EE-77-C-02-4442, July 1978.
- Witcofski, R. D., “Dispersion of flammable clouds resulting from large spills of liquid hydrogen,” NASA Technical Memorandum 83131, May 1981.
- Thomas, J. K., C. D. Eastwood and M. L. Goodrich, “Are unconfined hydrogen vapor cloud explosions credible?” Process Safety Progress, Vol. 34, Iss. 1, 2015.
- Health & Safety Laboratory, “IA HYSAFE & JRC IET Workshop—Research priorities and knowledge gaps in hydrogen safety,” Online: www.hsl.gov.uk
- Swain, M. R., P. A. Filoso and M. N. Swain, “An experimental investigation into the ignition of leaking hydrogen,” International Journal of Hydrogen Energy, Vol. 32, Iss. 2, 2007.
- Grune, J., M. Kuznetsov, A. Lelyakin and T. Jordan, “Spontaneous ignition processes due to high-pressure hydrogen release in air,” International Conference on Hydrogen Safety, 2011.
- Golub, V. V., D. I. Baklanov, T. V. Bazhenova, M. V. Bragin, S. V. Golovastav, M. T. Ivanov and V. V. Volodin, “Shock-induced ignition of hydrogen gas during accidental or technical opening of high-pressure tanks,” J. Loss Prev. Proc. Ind., Vol. 20, 2007.
- Thomas, J. K. and D. R. Malik, “Ammonia and hydrogen vapor cloud explosion testing (a tale of two gases),” AIChE, 62nd Annual Safety in Ammonia Plants and Related Facilities Symposium, Brooklyn, New York, September 10–14, 2017.
- Horn, B., O. Rodriguez, D. Malik, J. K. Thomas, B. Bang, Y. Kim and M. Lee, “Vented hydrogen DDTs,” 14th Global Congress on Process Safety, Orlando, Florida, April 22–25, 2018.
- Thomas, J. K., J. Geng, O. Rodriguez, S. Jallais, E. Vyazmina, D. Miller, B. Lindberg, R. Pawulski, “Potential for hydrogen DDT with ambient vaporizers,” Mary Kay O’Connor, Process Safety Symposium, College Station, Texas, October 23–25, 2018.
- Miller, D., C. D. Eastwood and J. K. Thomas, “Hydrogen jet vapor cloud explosion: Test data and comparison with predictions,” 11th Global Congress on Process Safety, Austin, Texas, April 27–29, 2015.
- Jallais, S., E. Vyazmina, D. Miller and J. K. Thomas, “Hydrogen jet vapor cloud explosion: A model for predicting blast size and application to risk assessment,” 13th Global Congress on Process Safety, San Antonio, Texas, March 26–29, 2017.
- Lowry, W. and M. Gandhi, “Performing PHAs for a hydrogen fuel cell test enclosure,” AIChE Center for Process Safety Europe Conference, Virtual Conference, October 20, 2020.
- Vilas, K. and R. J. Magraw, “Why proactive assessment of hydrogen fuelling risks is essential,” Hazards 30 IChemE, Virtual Conference, November 26–27, 2020.
- Moosemiller, M. and B. Galindo, “Hydrogen ignitions—Wildly differing opinions, and why everyone could be right,” 10th Global Congress on Process Safety, Loss Prevention Symposium, New Orleans, Louisiana, March 30–April 2, 2014.
MIKE MOOSEMILLER is a Senior Principal Consultant at BakerRisk. He has more than 30 yr of experience in risk management consulting to the process industries, and has authored or coauthored three books, including Guidelines for Determining the Probability of Ignition of a Released Flammable Mass, from the American Institute of Chemical Engineers. Prior to consulting, he worked for 7 yr as a commissioning engineer for new refining projects at UOP. He holds BS and MS degrees in chemical engineering from Purdue University and the University of Wisconsin–Madison, respectively.
KELLY THOMAS focuses primarily on the development and application of empirical, analytical and numerical models for the characterization of flammability and explosion phenomena. He also actively participates in the investigation of accidental industrial explosions, explosion consequence assessments, and testing. He has led explosion consequence assessments for refineries, chemical processing plants, offshore oil production platforms and other industrial facilities, as well as U.S. Department of Energy sites. Dr. Thomas has been involved with testing projects that include large-scale vapor cloud explosions, vented deflagrations, response of equipment to internal explosions and gas mixing within enclosures. He has published numerous papers and delivered many conference presentations related to these topics.

